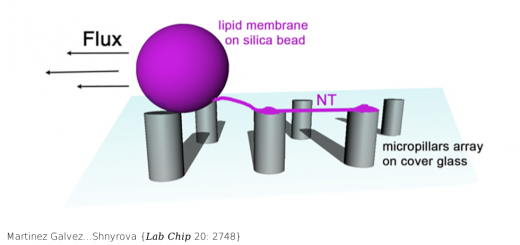
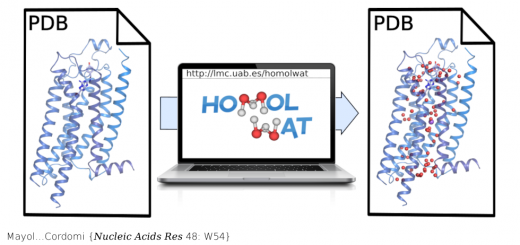
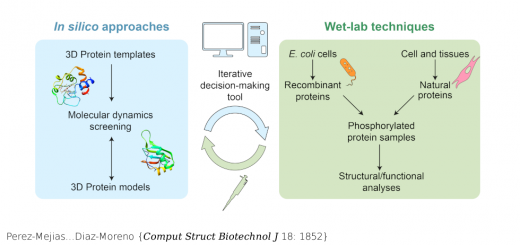
Schönfelder…Muñoz {Nat Commun 7: 11777}

Single molecule force spectroscopy reveals a highly complex mechanical unfolding of the Cold Shock Protein B from Thermotoga Maritima. This heterogeneous behaviour, shown here for the first time, contrasts with the simple two state unfolding suggested by traditional ensemble measurements on the same protein, using chemical denaturant.
A simple two-state protein unfolds mechanically via multiple heterogeneous pathways at single-molecule resolution
Schönfelder J, Perez-Jimenez R, Muñoz V.
Nat Commun 2016 Jun.; 7: 11777.
Amajor drive in protein folding has been to develop experimental technologies to resolve the myriads of microscopic pathways and complex mechanisms that purportedly underlie simple two-state folding behaviour. This is key for cross-validating predictions from theory and modern computer simulations. Detecting such complexity experimentally has remained elusive even using methods with improved time, structural or single-molecule resolution. Here, we investigate the mechanical unfolding of cold shock protein B (Csp), a showcase two-state folder, using single-molecule force-spectroscopy. Under controlled-moderate pulling forces, the unfolding of Csp emerges as highly heterogeneous with trajectories ranging from single sweeps to different combinations of multiple long-lived mechanical intermediates that also vary in order of appearance. Steered molecular dynamics simulations closely reproduce the experimental observations, thus matching unfolding patterns with structural events. Our results provide a direct glimpse at the nanoscale complexity underlying two-state folding, and postulate these combined methods as unique tools for dissecting the mechanical unfolding mechanisms of such proteins.
PubMed: 27248054. Doi: 10.1038/ncomms11777.