Viroporins:
A new target for fighting viral infections
 Over the last years an increasing number of viruses have jumped into the mass media and several names or acronyms (ebola, zika, SARS, MERS,…) have become familiar to the general public because of the high mortality rates associated with their infection in humans. The threat they pose to human life has to do mainly with the risk of infection spreading from the initial outbreak location to other countries and population sectors. Along with these pathogens, there are other viruses that remain a serious health problem, despite considerable therapeutic advances: The human immunodeficiency virus (HIV), responsible for the immunodeficiency syndrome (AIDS); the Hepatitis C virus (HCV), causing hepatitis C and some cancers; the influenza A virus (IAV); the Human respiratory syncytial virus (HRSV) a major cause of lower respiratory tract infections during infancy and childhood. This list is far from complete.
Over the last years an increasing number of viruses have jumped into the mass media and several names or acronyms (ebola, zika, SARS, MERS,…) have become familiar to the general public because of the high mortality rates associated with their infection in humans. The threat they pose to human life has to do mainly with the risk of infection spreading from the initial outbreak location to other countries and population sectors. Along with these pathogens, there are other viruses that remain a serious health problem, despite considerable therapeutic advances: The human immunodeficiency virus (HIV), responsible for the immunodeficiency syndrome (AIDS); the Hepatitis C virus (HCV), causing hepatitis C and some cancers; the influenza A virus (IAV); the Human respiratory syncytial virus (HRSV) a major cause of lower respiratory tract infections during infancy and childhood. This list is far from complete.
Viroporins
With the exception of the first two viruses just mentioned, all these pathogens share a common feature: They encode at least one protein with the characteristic ability to form ion channels or pores in some of the host cell membranes [Ewart et al. 1996, Henkel et al. 2010, Pavlovic et al. 2003, Pinto et al. 1992, Wilson et al. 2004, Surya et al. 2015, Lu et al. 2006, Gan et al. 2008], the preferred localization being the endoplasmic reticulum (ER), the Golgi apparatus and the plasma membrane. These proteins were coined as viroporins because of their similarity to the ion channels commonly known as porins. In fact, the vast majority of viroporins are simple, poorly specific hydrophilic pores, often lacking any voltage-dependence or gating mechanism. The existence of viroporins was proposed nearly forty years ago after the observation of enhanced membrane permeability in virus-infected cells [Nieva et al. 2012, Carrasco, 1978]. Although they are usually small hydrophobic proteins (less than 100 amino acids) they exhibit marked differences in their tertiary structure, known at atomic resolution only in a few cases. They have at least one amphipathic helix that constitutes its transmembrane domain (TM). In fact, they have been classified according to the number of TMs and their topology (Figure 1). Viroporin self-assembly and oligomerization, commonly tetrameric, pentameric, hexameric or even heptameric, is a requisite for ion channel formation. From the initial identification of the first viroporins, electrophysiology has been a valuable tool for virologists in their attempts to unravel the significance and biological role of the ion channel activity in the viral life cycle. The viroporin field is relatively new and opens a wealth of interesting questions to ion channel biophysicist too. Here is a brief summary of the accumulated evidence of the relevance of viroporin ion channel (IC) activity and some of the key observations following electrophysiological studies of a few viroporins in model membranes.

Figure 1. Classification of viroporins according to the number of TMs and the membrane topology of the constituent monomers. Clasees are named I or II for single or double membrane-spanning domains, respectively. Recent evidence of a viroporin with 3 TMs (protein 3a from SARS-CoV) would update this scheme to account for a new third Class. Reprinted by permission from Macmillan Publishers Ltd: Nieva, Madan & Carrasco. Nat Rev Microbiol 2012, 10: 563, copyright 2012.
Relevance of viroporin ion channel activity
The cell homeostasis involves a tightly regulated balance of the concentration of the main monovalent (K+, Na+ and Cl–) and divalent (mainly Ca2+) ions and protons in the subcellular compartments and the extracellular media. Therefore, any viroporin-induced change of the asymmetric distribution of ions between different cell organelles may be crucial. Viroporins may modify the electrochemical gradients that are essential for proper cell functioning [Dubyak, 2004]. Of particular importance for cell function is keeping the Ca2+ concentration at its optimum level in each compartment. Given that there are differences of two to three orders of magnitude in Ca2+ concentration between the ER and the cytosol, it is expected that some viroporins like SARS-CoV-E [Nieto-Torres, 2015a] and the Rotavirus NSP4 [Pham et al. 2017], which are Ca2+ conducting channels, may disrupt the cellular Ca2+ homeostasis. The loss of ion homeostasis may lead to stress responses and even to apoptosis [Nieva et al. 2012, Madan et al 2008, Bhowmick et al. 2012]. In some cases the cell response involves the activation of a macromolecular complex called the inflammasome, key in the stimulation of innate immunity [Nieto-Torres et al. 2014].

Figure 2. Pathways stimulated by viroporin ion channel activity leading to pathology. Molecular patterns associated with viral infections are recognized by cellular sensors (signal 1), which activate the transcription and translation of the NLRP3 inflammasome components (NLRP3, ASC and procaspase-1) and the inactive pro-IL-1β. Viroporins inserted in the intracellular organelles, such as the ER or the Golgi apparatus, favor the leakage of Ca2+ and H+ ions that move following their electrochemical gradient into the cell cytoplasm. This ionic imbalance (signal 2) induces the assembly of the inflammasome complex, which triggers the maturation of pro-IL-1β into IL-1β through the action of caspase-1. Secreted IL-1β mediates a potent pro-inflammatory response that can be deleterious for the cell and the organism, when overstimulated. In addition, alteration of ionic milieus in intracellular compartments comes along with a protein transport delay or blockage. This results in a decrease of the levels of MHC-I molecules (blue rectangles) at the plasma membrane, preventing the infected cell to be recognized by the immune system. Protein transport blockage also diminishes the levels and activity in the cell surface of ion channels and transporters, crucial in the resolution of edema accumulation. Reprinted under CC-BY license from Nieto-Torres, et al. Viruses 2015, 7: 2786.
Virologists have reported various effects of viroporin IC activity, not only from in vitro assays but also from several in vivo experiments with mice. Firstly, viroporins stimulate key aspects of the viral cycle such as entry, assembly, trafficking and release of viral particles. In general, viroporin defective viruses exhibit much lower viral yields. They also favor virus propagation, as has been shown after pharmacological inhibition of the viroporin ion conductance in the IAV M2 protein. In addition, viroporins are also involved in pathogenesis. (Figure 2) shows several pathways stimulated by viroporin IC activity leading to pathology. In fact, some viroporin-deleted viruses have been used as potential vaccine candidates [Nieto-Torres, 2015a]. A recent study that combined in vitro and in vivo experiments proved that animals infected with the SARS-CoV lacking E protein IC activity showed a reduced mortality in comparison with those inoculated with the parental virus [Nieto-Torres et al. 2014].
In view of the relevance of viroporin IC activity in viral production, propagation and pathogenesis, these protein channels represent a promising target for combined therapeutic interventions [Hyser 2015, Scott & Griffin 2015]. This has motivated the search for inhibitors of the IC activity. A number of compounds that interfere with viroporins IC activity have been reported and assayed in model membranes and sometimes in cell cultures, but just a few are adequate for pharmacological use. Amantadine was the first inhibitor approved for humans, and it has been used for around 20 years in the treatment of IAV infections [Oxford 2007] since it binds to the M2 protein, blocking ion conductance.

Figure 3. The crystal structure of the transmembrane proton channel domain of the influenza A M2 protein. The model shows tetrameric arrangement of helices with a channel-blocking drug, amantadine, located in the center (red). Rendered from PDB ID 3C9J, published in Thomaston et al. 2015. By Opabinia regalis – own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=51193047.
Studying viroporins at the single-channel level: lessons we learn
Electrophysiological techniques are a valuable tool to investigate the IC properties of viroporins, using their different variants: Planar lipid bilayer, liposome patch-clamp, whole-cell patch clamp, and liposome swelling assays. All of them provide useful information about the size of the solutes able to permeate across viroporins, the influence of the membrane composition on IC formation, the external conditions (pH, ionic concentrations) that modulate the IC conductance and selective properties, etc.
The lack of knowledge about the tertiary structure of most viroporins at atomic resolution makes electrophysiological measurements even more necessary, particularly if one wants to pinpoint the amino acid residues that are essential for viroporin assembly, oligomerization and IC activity. The analysis and manipulation of viroporin IC properties at the molecular level represents a new challenge for the collaboration between virologists and biophysicists, to benefit from synergies between their respective expertise. Note that, in general, electrophysiology equipment and skills are not a typical part of the virology lab. Over the recent years a lot of useful information has been gathered, but there is still much more to learn in the future.
Given the difficulty of using the purified full protein, evidence of identical results (conductance, selectivity, oligomerization state) in terms of IC activity using the viroporin and a peptide with the protein TM domain amino acid sequence is extremely important [Verdiá-Báguena et al. 2012, 2013]. Also, at a general level, it has been reported that channel properties examined in giant unilamellar vesicles and planar lipid bilayers are fully consistent with each other [Largo et al. 2016].
As viroporins localize in different membranes that vary in lipid composition, it is essential to test the influence of the membrane lipid composition on IC properties. Experiments with lipids that induce membrane negative curvature (like phosphatidylethanolamine, PE) yield lower probability of SARS-CoV E channel formation [Verdiá-Báguena et al. 2012]. In addition, whenever a negatively charged lipid takes part in membrane composition, single-channel conductance and ion selectivity depart substantially from the values measured in neutral lipid membranes (Figure 4). Both facts are consistent with SARS-CoV E viroporin being a protein-lipid pore, i.e. a dynamic structure in which assembled TM helices are combined with lipid molecules to form a hydrophilic pore. This trait is probably characteristic of many more viroporins.
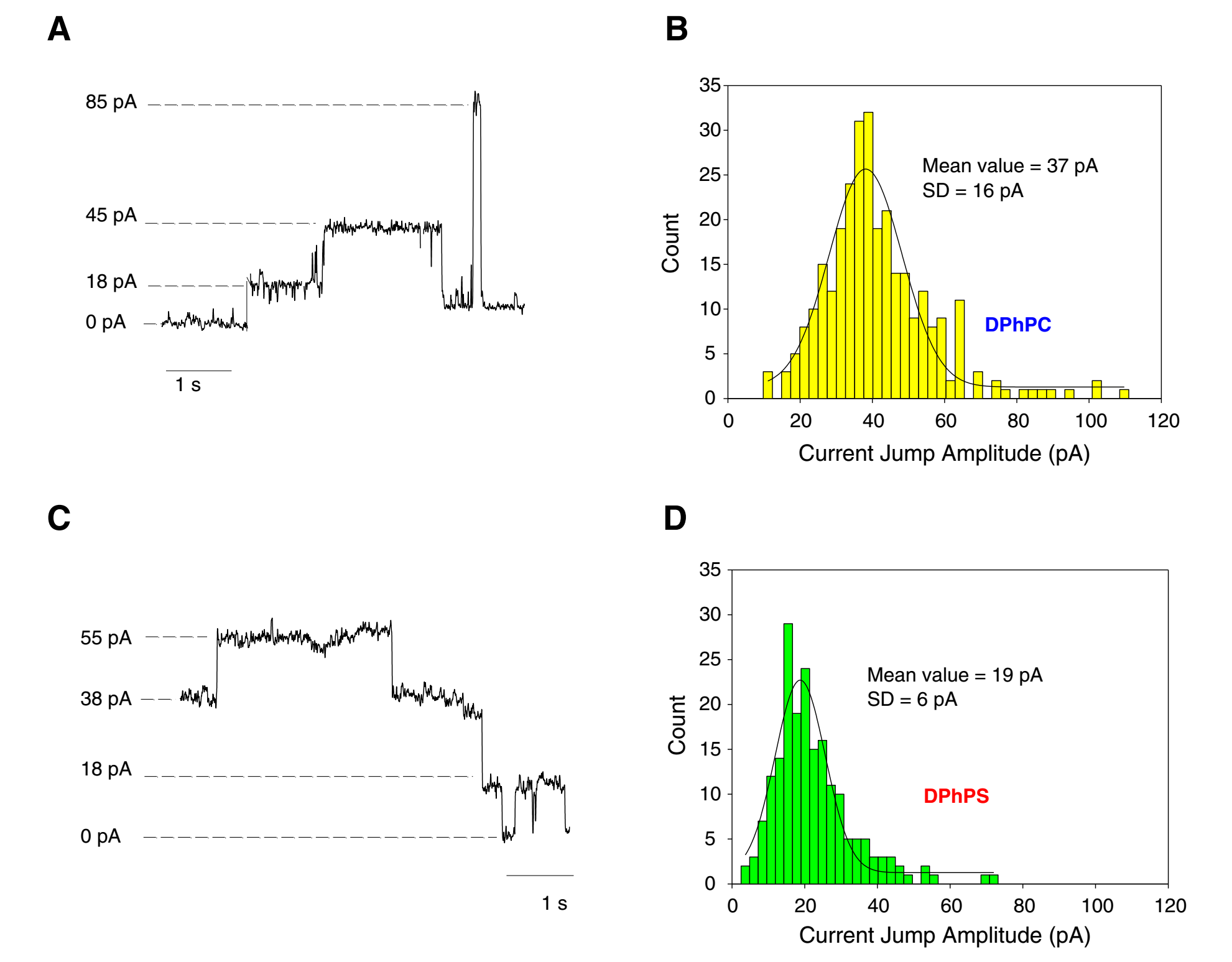
Figure 4. SARS-CoV E channel current recordings and histograms of the current jump amplitude in 1 M KCl at pH 6. A and B: Traces and histogram of channels formed in neutral DPhPC membranes. C and D: Traces and histogram of channels formed in negatively charged DPhPS membranes. As the corresponding histograms show, the current jump levels in DPhPS membranes are better defined than in DPhPC, where a larger variety of current jump amplitudes are recorded. Reprinted from Verdiá-Báguena, et al. BBA-Biomembranes 2013, 1828: 2026. copyright 2013, with permission from Elsevier.
Apart from the IAV M2 protein, which is highly selective for protons, most other viroporins usually show mild ion selectivity, meaning that in general these channels do not display preference for a particular ion. Still, this general statement is a source of misconceptions because channel selectivity is often strongly dependent on a number of factors alien to the channel itself, like ion concentrations, pH, the host membrane composition, etc. Figure 5A illustrates this fact with reversal potential measurements performed with the SARS-CoV E channel. This is the potential difference needed to get zero electric current when there is an ionic concentration gradient across the channel, and is the usual measure of channel ion selectivity.

Figure 5. pH modulation of SARS-CoV E protein channel selectivity in neutral and charged lipid membranes in monovalent ions (KCl) and divalent salt (CaCl2) solutions. (A) The reversal potential was measured in asymmetric (500/50 mM) KCl solutions in neutral DPhPC (red circles) and negatively charged DPhPS membranes (green squares). In a third series of measurements with DPhPS membranes, 15 mM CaCl2 were added on both KCl solutions (orange squares). In KCl solutions, the channel behaves as an almost perfect cation selective pore at neutral pH in charged membranes, whereas in neutral membranes it barely selects cations over anions. A small amount of CaCl2 reduces the cationic selectivity. In addition, the change of reversal potential upon acidification of the solution reflects two consecutive titrations (at pH ~4 and pH ~1.5) in charged membranes and KCl solutions (green squares), in contrast with a single titration in the other two series. (B) The cartoon outlines a protein-lipid pore where lipid head groups (cyan) are oriented towards the pore, modulating ion conductance and selectivity. Panel A Reprinted from Nieto-Torres et al. Virology 2015, 485: 330. copyright 2015, with permission from Elsevier. Panel B Reprinted under CC-BY license from Nieto-Torres et al. Viruses 2015, 7: 2786.
Viroporins might look at first sight rather simple, passive diffusion, hydrophilic pores, almost unable to discriminate between small ions. However, even a single mutation in one amino acid is enough to abolish IC activity [Nieto-Torres et al. 2014]. As soon as the atomic structure of more viroporins is resolved, a more efficient search for inhibitors will be possible.
Conclusions and future prospects
Unlike many other channels with key neurophysiological functions, the viroporin research field is still in its infancy. So far, there is solid evidence that they are key contributors to virus propagation and stimulators of pathogenesis, but the specific mechanisms linking IC activity with the virus life cycle need to be disclosed in most viroporins. Understanding the molecular and physicochemical structure of these protein channels is a first step towards the rational design of specific IC activity inhibitors and strategies to fight viral infection. The interaction between the viroporin TM domain and the membrane in terms of the lipid charge and lamellar or non-lamellar phase is not completely understood yet. In any case, viroporins emerge as excellent candidates for development of novel antiviral therapies.
Lab Molecular Biophysics (Department of Physics),
UJI – Universitat Jaume I, Castellón (Spain).
References
Bhowmick R, Halder UC, Chattopadhyay S, Chanda S, Nandi S, Bagchi P, Nayak MK, Chakrabarti O, Kobayashi N, Chawla-Sarkar M. “Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection”. J Biol Chem 2012, 287: 35004. DOI: 10.1074/jbc.M112.369595.
Carrasco L. “Membrane leakiness after viral infection and a new approach to the development of antiviral agents”. Nature 1978, 272: 694. DOI: 10.1038/272694a0.
Dubyak GR. “Ion homeostasis, channels, and transporters: An update on cellular mechanisms”. Adv Physiol Educ 2004, 28: 143. DOI: 10.1152/advan.00046.2004.
Ewart GD, Sutherland T, Gage PW, Cox GB. “The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels”. J Virol 1996, 70: 7108. PMCID: PMC190763.
Gan SW, Ng L, Lin X, Gong X, Torres J. “Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain”. Protein Sci 2008, 17: 813. DOI: 10.1110/ps.073366208.
Henkel M, Mitzner D, Henklein P, Meyer-Almes FJ, Moroni A, Difrancesco ML, Henkes LM, Kreim M, Kast SM, Schubert U, Thiel G. “The proapoptotic influenza A virus protein PB1-F2 forms a nonselective ion channel”. PLoS One 2010, 5: e11112. DOI: 10.1371/journal.pone.0011112.
Hyser JM. “Viroporins”, in Electrophysiology of Unconventional Channels and Pores (Delcour AH, Ed.), 153–181, Springer, Switzerland, 2015.
Largo E, Verdiá-Báguena C, Aguilella VM, Nieva JL, Alcaraz A. “Ion channel activity of the CFSV p7 viroporin in surrogates of the ER lipid bilayer”. BBA Biomembranes 2016, 1858: 30. DOI: 10.1016/j.bbamem.2015.10.007.
Lu W, Zheng BJ, Xu K, Schwarz W, Du L, Wong CK, Chen J, Duan S, Deubel V, Sun B. “Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release”. Proc Natl Acad Sci USA 2006, 103: 12540. DOI: 10.1073/pnas.0605402103.
Madan V, Castello A, Carrasco L. “Viroporins from RNA viruses induce caspase-dependent apoptosis”. Cell Microbiol 2008, 10: 437. DOI: 10.1111/j.1462-5822.2007.01057.x.
Nieto-Torres JL, Dediego ML, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Fernandez-Delgado R, Castaño-Rodriguez C, Alcaraz A, Torres J, Aguilella VM, Enjuanes L. “Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis”. PLoS Pathog 2014, 10: e1004077. DOI: 10.1371/journal.ppat.1004077.
Nieto-Torres JL, Verdiá-Báguena C, Castaño-Rodriguez C, Aguilella VM. Enjuanes L. “Relevance of viroporin ion channel activity on viral replication and pathogenesis”. Viruses 2015a, 7: 3552. DOI: 10.3390/v7072786.
Nieto-Torres JL, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Castano-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L. “Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome”. Virology 2015b, 485: 330. DOI: 10.1016/j.virol.2015.08.010.
Nieva JL, Madan V, Carrasco L. “Viroporins: Structure and biological functions”. Nat Rev Microbiol 2012, 10: 563. DOI: 10.1038/nrmicro2820.
Oxford JS. “Antivirals for the treatment and prevention of epidemic and pandemic influenza”. Influenza Other Respir Viruses 2007, 1: 27. DOI: 10.1111/j.1750-2659.2006.00006.x.
Pavlovic D, Neville DC, Argaud O, Blumberg B, Dwek RA, Fischer WB, Zitzmann N. “The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives”. Proc Natl Acad Sci USA 2003, 100: 6104. DOI: 10.1073/pnas.1031527100.
Pham T, Perry JL, Dosey TL, Delcour AH, Hyser JM. “The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel”. Sci Rep 2017, 7: 43487. DOI: 10.1038/srep43487.
Pinto LH, Holsinger LJ, Lamb RA. “Influenza virus M2 protein has ion channel activity”. Cell 1992, 69: 517. DOI: 10.1016/0092-8674(92)90452-I.
Scott C, Griffin S. “Viroporins: structure, function and potential as antiviral targets”. J Gen Virol. 2015, 96: 2000. DOI: 10.1099/vir.0.000201.
Surya W, Li Y, Verdia-Baguena C, Aguilella V.M, Torres J. “MERS coronavirus envelope protein has a single transmembrane domain that forms pentameric ion channels”. Virus Res 2015, 201: 61. DOI: 10.1016/j.virusres.2015.02.023.
Thomaston JL, Alfonso-Prieto M, Woldeyes RA, Fraser JS, Klein ML, Fiorin G, DeGrado WF. “High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction”. Proc Natl Acad Sci USA 2015, 112: 14260. DOI: 10.1073/pnas.1518493112.
Verdiá-Báguena C, Nieto-Torres JL, Alcaraz A, Dediego ML, Torres J, Aguilella VM, Enjuanes L. “Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids”. Virology 2012, 432: 485. DOI: 10.1016/j.virol.2012.07.005.
Verdiá-Báguena C, Nieto-Torres JL, Alcaraz A, Dediego ML, Enjuanes L, Aguilella VM. “Analysis of SARS-CoV E protein ion channel activity by tuning the protein and lipid charge”. Biochim Biophys Acta 2013, 1828: 2026. DOI: 10.1016/j.bbamem.2013.05.008.
Wilson L, McKinlay C, Gage P. “SARS coronavirus E protein forms cation-selective ion channels”. Virology 2004, 330: 322. DOI: 10.1016/j.virol.2004.09.033.
