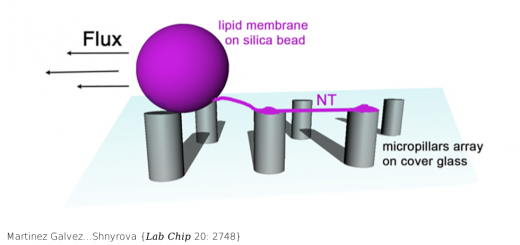
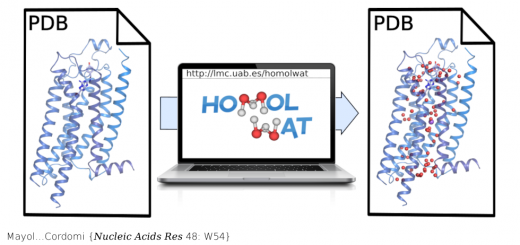
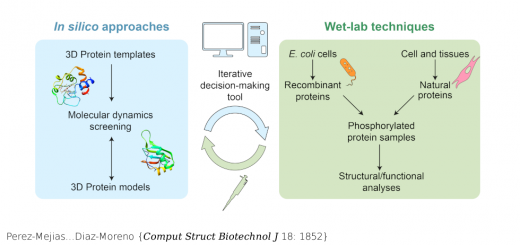
Unione L, Lenza MP, Arda A, Urquiza P, Lain A, Falcon-Perez JM, Jimenez-Barbero J, Millet O.
ACS Cent Sci 2019 Sep; 5: 1554.
Protein N-glycosylation stands out for its intrinsic and functionally related heterogeneity. Despite its biomedical interest, Glycoprofile analysis still remains a major scientific challenge. Here, we present an NMR-based strategy to delineate the N-glycan composition in intact glycoproteins and under physiological conditions. The employed methodology allowed dissecting the glycan pattern of the IgE high-affinity receptor (FcepsilonRIalpha) expressed in human HEK 293 cells, identifying the presence and relative abundance of specific glycan epitopes. Chemical shifts and differences in the signal line-broadening between the native and the unfolded states were integrated to build a structural model of FcepsilonRIalpha that was able to identify intramolecular interactions between high-mannose N-glycans and the protein surface. In turn, complex type N-glycans reflect a large solvent accessibility, suggesting a functional role as interaction sites for receptors. The interaction between intact FcepsilonRIalpha and the lectin hGal3, also studied here, confirms this hypothesis and opens new avenues for the detection of specific N-glycan epitopes and for the studies of glycoprotein-receptor interactions mediated by N-glycans.
PubMed: 31572782. Doi: 10.1021/acscentsci.9b00540. Free PMC