Structural Evidence of Amyloid Fibril Formation in the Putative Aggregation Domain of TDP-43
Mompeán M, Hervás R, Xu Y, Tran TH, Guarnaccia C, Buratti E, Baralle F, Tong L, Carrión-Vázquez M, McDermott AE, Laurents DV.
J Phys Chem Lett. 2015 Jul; 6: 2608 [Epub 22 Jun 2015].
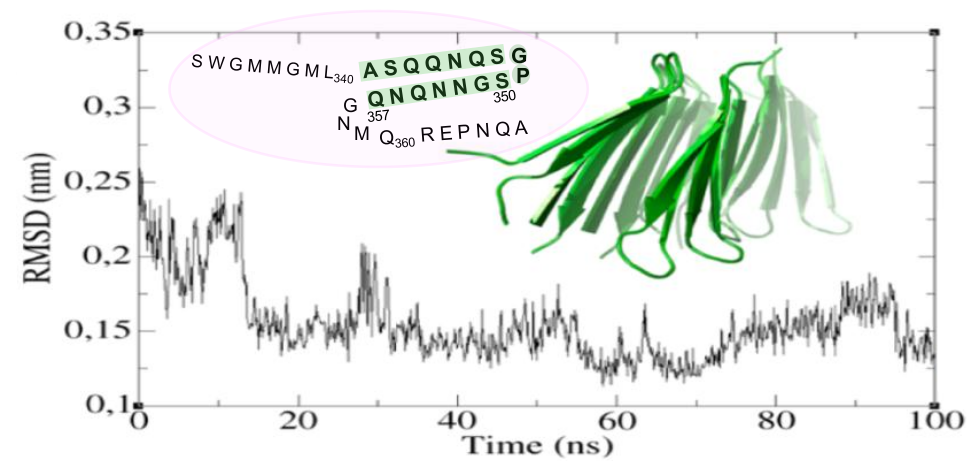
TDP-43 can form pathological proteinaceous aggregates linked to ALS and FTLD. Within the putative aggregation domain, engineered repeats of residues 341-366 can recruit endogenous TDP-43 into aggregates inside cells; however, the nature of these aggregates is a debatable issue. Recently, we showed that a coil to β-hairpin transition in a short peptide corresponding to TDP-43 residues 341-357 enables oligomerization. Here we provide definitive structural evidence for amyloid formation upon extensive characterization of TDP-43(341-357) via chromophore and antibody binding, electron microscopy (EM), solid-state NMR, and X-ray diffraction. On the basis of these findings, structural models for TDP-43(341-357) oligomers were constructed, refined, verified, and analyzed using docking, molecular dynamics, and semiempirical quantum mechanics methods. Interestingly, TDP-43(341-357) β-hairpins assemble into a novel parallel β-turn configuration showing cross-β spine, cooperative H-bonding, and tight side-chain packing. These results expand the amyloid foldome and could guide the development of future therapeutics to prevent this structural conversion.
PubMed: 26266742. Doi: 10.1021/acs.jpclett.5b00918