Experimental measurement of binding energy, selectivity, and allostery using fluctuation theorems
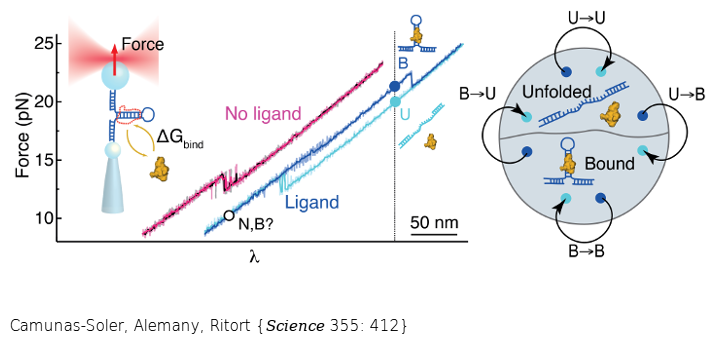
A fluctuation theorem for ligand binding allows to extract binding energies from non-equilibrium force experiments where macromolecules are pulled apart. The measurement does not require dilute ligand conditions or to assume a reaction model of the interaction.
Camunas-Soler J, Alemany A, Ritort F.
Science 2017 Jan.; 355: 412.
Thermodynamic bulk measurements of binding reactions rely on the validity of the law of mass action and the assumption of a dilute solution. Yet, important biological systems such as allosteric ligand-receptor binding, macromolecular crowding, or misfolded molecules may not follow these assumptions and may require a particular reaction model. Here we introduce a fluctuation theorem for ligand binding and an experimental approach using single-molecule force spectroscopy to determine binding energies, selectivity, and allostery of nucleic acids and peptides in a model-independent fashion. A similar approach could be used for proteins. This work extends the use of fluctuation theorems beyond unimolecular folding reactions, bridging the thermodynamics of small systems and the basic laws of chemical equilibrium.
PubMed: 28126820. Doi: 10.1126/science.aah4077.