Cordeiro…Pons {JBC 290: 21200}

The interdigitation of charged side chains of three proteins is the key for a highly specific electrostatic interaction. Electrostatics also determine the intrinsic dynamics of one of the components, possibly needed for the formation and dissociation of specific complexes.
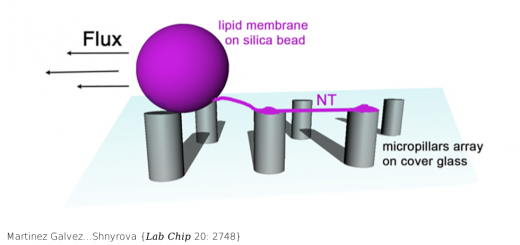
A Three-protein Charge Zipper Stabilizes a Complex Modulating Bacterial Gene Silencing
Cordeiro TN, García J, Bernadó P, Millet O, Pons M.
J Biol Chem. 2015 Aug; 290: 21200 [Epub 17 June 2015].
The Hha/YmoA nucleoid-associated proteins help selectively silence horizontally acquired genetic material, including pathogenicity and antibiotic resistance genes and their maintenance in the absence of selective pressure. Members of the Hha family contribute to gene silencing by binding to the N-terminal dimerization domain of H-NS and modifying its selectivity. Hha-like proteins and the H-NS N-terminal domain are unusually rich in charged residues, and their interaction is mostly electrostatic-driven but, nonetheless, highly selective. The NMR-based structural model of the complex between Hha/YmoA and the H-NS N-terminal dimerization domain reveals that the origin of the selectivity is the formation of a three-protein charge zipper with interdigitated complementary charged residues from Hha and the two units of the H-NS dimer. The free form of YmoA shows collective microsecond-millisecond dynamics that can by measured by NMR relaxation dispersion experiments and shows a linear dependence with the salt concentration. The number of residues sensing the collective dynamics and the population of the minor form increased in the presence of H-NS. Additionally, a single residue mutation in YmoA (D43N) abolished H-NS binding and the dynamics of the apo-form, suggesting the dynamics and binding are functionally related.
PubMed: 26085102. Doi: 10.1074/jbc.M114.630400